when atoms share electrons unequally the bond formed is a
Click to see full answer Beside this what type of bond is formed when atoms share electrons. A polar covalent bond is formed when the electrons are shared unequally.
 |
| 9 3 Covalent Bonding Chemistry Libretexts |
Electronegativity EN is a measure of an atoms ability to.
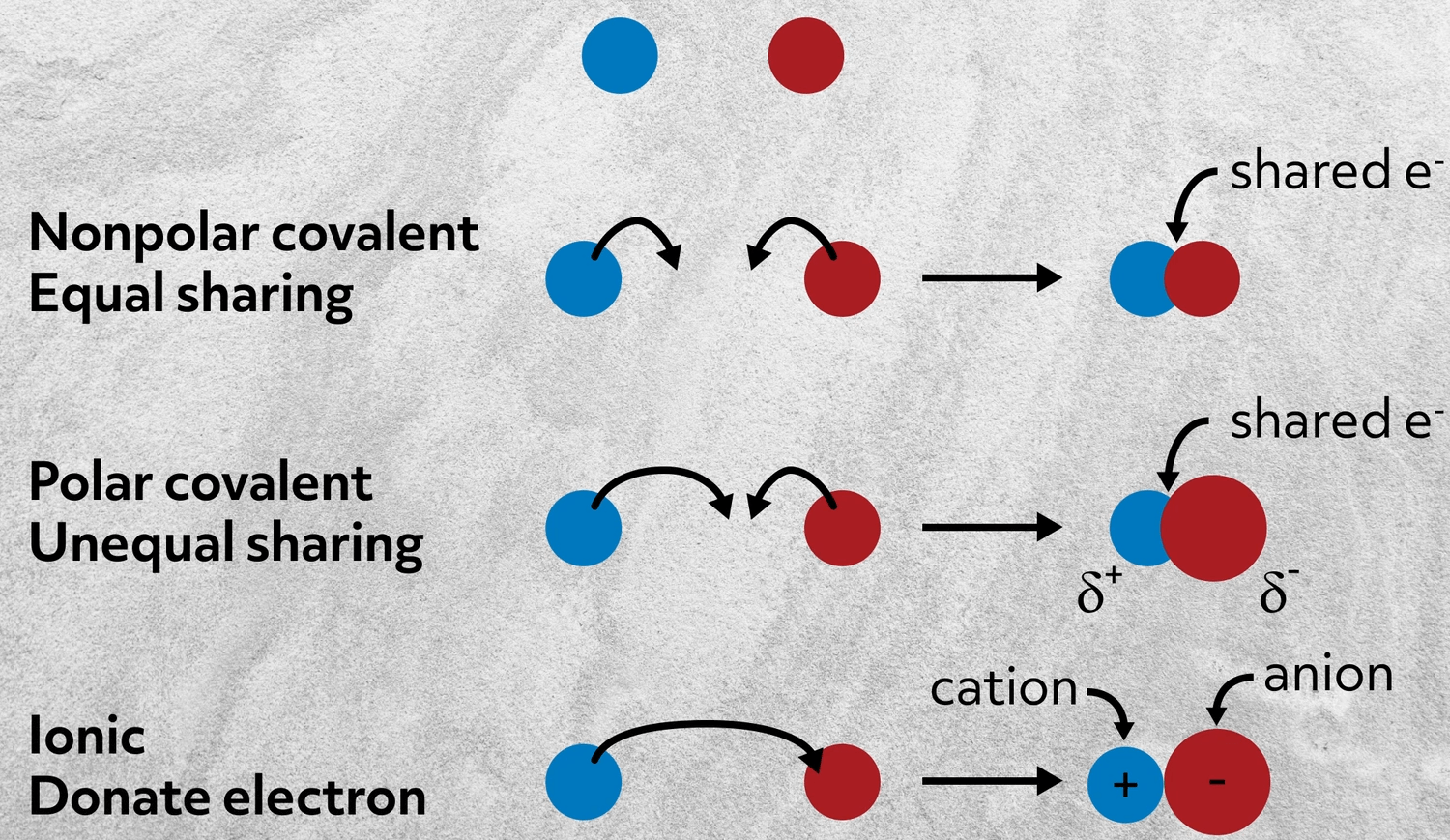
. 1 When Electrons Are Unequally Shared Between Two Atoms In A Bond The Bond Is Said To Be. When atoms share electrons unequally the bond formed is a. Polar refers to the two extremes of like unlike poles of a magnet. A compound is made when atoms of two or more elements bond in a chemical reaction.
When two pairs of electrons are shared between two atoms a ____ bond is formed. Isotopes of the same element differ from each other in the number of _____. Word Craze is the best version of puzzle word games at the moment. A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share.
Click to see full answer Also asked what type of bond is formed when atoms share electrons. What happens to electrons in an covalent bond. How do the atoms share electrons in molecule formation so that each atom appears to have a noble gas electron. What is formed when atoms of two or more elements bond in a chemical reaction.
Covalent bonding occurs when pairs of electrons are shared by atoms. Bond Strength Chemistry LibreTexts. For older puzzles we recommend you to visit the archive page over at Word Craze Daily Puzzle Answers. When atoms differ in electronegativity the most electronegative atom will draw the shared electrons more strongly to itself.
A ____ bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. The bond is known to be polar covalent if there is an electronegativity difference between the. Two hydrogen atoms and one oxygen atom bond to form a molecule of water H 2 O. Polar covalent bonding occurs because one atom has a stronger affinity preference for electrons than the other yet.
Much like the poles on a mini magnet the atoms connected by a polar bond become positive and negative poles. Types of Chemical Bonds Polar Covalent Bond electrons unequally shared by atoms unsymmetrical Nonpolar Covalent Bond electron pairs are equally shared by atoms symmetrical Ionic Bond transfer of electrons from metallic to non-metallic to form ions Metallic Bond force of attraction between valence electrons and. A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
455 4 votes. When atoms share electrons unequally the bond formed is an_____bond. This potential will make the resulting molecule slightly polar allowing it to form weak bonds with other polar molecules. 2 What type of bond is formed when electrons are not equally shared between two atoms.
3 When bonding electrons are shared unequally the bond that is formed is nonpolar covalent. In this post we have shared the answer for ______ bonds are formed when atoms share electrons. The shared electrons are typically near the middle of the bond between the 2 atoms in a covalent bond. A Polar Covalent Bond is created when the shared electrons between atoms are not equally shared.
When atoms share electrons unequally the bond formed is. A polar bond is formed when electrons are unequally shared between two atoms. What are two charged particles. The solution we have below has a total of 8 Letters.
Why do atoms share electrons unequally in a covalent bond. 4 When atoms share electrons. When this happens we usually say that the electrons are shared unequally between the atoms. 20_______________ bonds are formed when atoms share electrons unequally.
The electron arrangement of the outer energy level of an atom determines whether or not it will form chemical bonds. As a result one end of the molecule has a slightly negative charge and the other a slightly positive charge. In pure covalent bonds the electrons are shared equally. A bond in which electrons are shared unevenly is known as a polar bond.
This occurs when one atom has a higher electronegativity than the atom it is sharing with.
 |
| What Is An Ionic Bond Video |
 |
| Chemical Bonds Anatomy And Physiology |
 |
| Ionic And Covalent Bonds Overview |
 |
| Ionic And Covalent Bonds Overview |
 |
| 3 Covalent Bonds In Which Atoms Share Electrons Unequally Are Polar Labxchange |
Posting Komentar untuk "when atoms share electrons unequally the bond formed is a"